 North Louisiana’s first Micra™ AV pacemaker, the world’s smallest pacemaker with atrioventricular (AV) synchrony, was implanted by Basel Kasabali, MD, cardiovascular disease specialist with Willis-Knighton Cardiology, on Monday, April 20. The procedure was performed in the electrophysiology lab in the Willis-Knighton Heart & Vascular Institute.
North Louisiana’s first Micra™ AV pacemaker, the world’s smallest pacemaker with atrioventricular (AV) synchrony, was implanted by Basel Kasabali, MD, cardiovascular disease specialist with Willis-Knighton Cardiology, on Monday, April 20. The procedure was performed in the electrophysiology lab in the Willis-Knighton Heart & Vascular Institute.
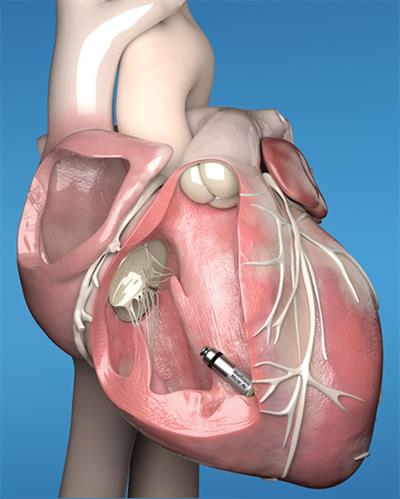
The device, comparable in size to a large vitamin and about one-tenth the size of a traditional pacemaker, is a new treatment option for patients with AV block. AV block is a disorder that occurs when the electrical signals between the chambers of the heart (the atria and the ventricles) are impaired. Pacemakers are the most common way to treat AV block, helping restore the heart’s normal rhythm and relieve symptoms by coordinating the electrical activity of the atria and the ventricles.
Historically, patients with AV block have been treated with traditional dual-chamber pacemakers that are implanted in the upper chest, under the skin below the collar bone, and connected by wires to the heart. Identical in size and shape to the original Micra Transcatheter Pacing System (TPS) approved in 2016, Micra™ AV requires neither wires nor placement below the skin. It has several additional internal atrial sensing algorithms, which enable it to adjust and synchronize pacing in the heart’s upper and lower chambers.
Physicians at Willis-Knighton have elected to use Medtronic’s Micra™ AV based on its ability to deliver therapy via a minimally invasive approach. During the implant procedure, the device is inserted through a catheter and implanted directly into the heart, eliminating chest incision, scar or bump that results from conventional pacemakers.
“The new Micra™ AV leadless pacemaker is a game changer,” Dr. Kasabali said. “Some patients who may have needed a pacemaker may have been unable to receive a pacemaker due to circumstances, and now half of the patients who need a pacemaker will be eligible for the new Micra AV.
“Most importantly, though, the Micra™ AV pacemaker greatly reduces the potential for complications that are common with traditional techniques.”
Micra™ AV was approved by the U.S. Food and Drug Administration in January 2020.